Oxidative Phosphorylation And The ETC
Establishment of proton gradient
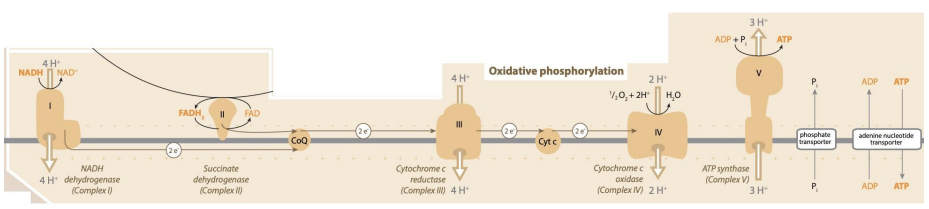
Complex I: NADH dehydrogenase
NADH passes 2 electrons to FMN (derivative of Riboflavin, vitamin B2) forming NAD+. Patients deficient in vitamin B2 will struggle with metabolism.
The 2 electrons are used to pump 4 protons into the intermembrane space (or mitosol).
Result
In:
- NADH (2 electrons)
Out:
- NAD+
- 2 electrons (to charge a Coenzyme Q)
- 4 H+ (to intermembrane space)
Complex II: Succinate dehydrogenase
This is a bifunctional enzyme from the Krebs cycle. It does not pump protons.
FADH will pass 2 electrons to charge a Coenzyme Q (along with Complex I) forming FAD+. Coenzyme Q is also known as Ubiquinone.
Result
In:
- FADH (2 electrons)
Out:
- FAD+
- 2 electrons (to Coenzyme Q)
Complex III: Cytochrome C reductase
Coenzyme Q (CoQ) passes the electrons from CI/II which are used to pump 4 protons into the intermembrane space. The 2 electrons are then passed to cytochrome C.
Cytochrome C
Cytochrome C (CytoC) functions as a rate limit to chain action. This prevents immediate conversion from reactants to products, and insures the inner membrane does not become too acidic with over transport of protons.
Cyanide poisoning
Cyanide binds to the F3+ heme of Cytochrome C, preventing electron transport to Complex IV. This creates electron buildup, deactivating the other components, and destroys the proton gradient.
The treatment is IV NaNO2 or inhaled Amyl Nitrite. These convert Fe2+ into Fe3+ in some hemoglobin, which sucks up the cyanide from CytoC. An abundance of normal hemoglobin Fe2+ will continue the chain.
Result
In:
- Coenzyme Q (2 electrons)
Out:
- 4 H+ (to intermembrane space)
- 2 electrons (to cytochrome C)
Complex IV: Cytochrome C oxidase
Electrons from CytoC are passed to oxygen (forming H2O) and 2 protons are pumped into the intermembrane space. This is the reason we breathe oxygen.
This step is exergonic (free energy higher in reactants). It has to be because it is at the end of the chain, the inner membrane space is saturated with protons. The transfer to of electrons to oxygen is super exergonic, pushing the other reaction forward.
Result
In:
- Cytochrome C (2 electrons)
- Oxygen (O2)
Out:
- 2 protons (to intermembrane space)
- Water (H2O)
Oxidative phosphorylation
ATP synthase thermodynamics
The high levels of protons pumped into the inner membrane space of the mitochondria makes the reaction to get them out exergonic.
ATP synthase is, therefore, enthalpically and entropically favorable. The dS is positive because the protons are all sorted together in the inner membrane space and the dH is negative because a lot of work was required to move the charges together.
Making ATP
ADP
ADP is brought into the inner membrane space via Adenine Nucleotide Translocase. It is an antiporter which exchanges an ATP4- in the matrix for an ADP3-. The ATP4- is attracted to the protons in the inner membrane space. This process also reduces the charge of the matrix (-1 for each ADP) and promotes the movement of H+ into the matrix.
Phosphate
Phosphate is brought into the matrix via Phosphate Translocase. It is a symporter which brings a proton from the intermembrane space (down its concentration gradient) into the matrix with the phosphate.
Reaction ratios
For each 1 ATP in ATP synthase, 4 H+ are pumped into the matrix
10 total protons are pumped into the inner membrane space over the chain, which generates 2.5 ATP.
Because only one NADH molecule is oxidized per cycle, 1 NADH = 2.5 ATP
Similarly, FADH2 allows 6 H+ to be pumped (starting at Complex II), which would result in 1.5 ATP. Therefore, 1 FADH2 = 1.5 ATP.