Osmosis And Fluid Shifts
Movement across semi-permeable membrane
If a solute is more concentrated in an area but cannot move across a membrane according to its concentration gradient, then water will move across instead. This is measurable as osmotic pressure.
Osmotic pressure
Depends on concentration of particles:
Where pi is osmotic pressure, c is concentration, R is the gas constant, T is the temperature (in k) of the fluid.
i is the dissociation number (how many particles upon dissolving)
sigma is the reflection coefficient, a measure of permeability to water. therefore:
= highly permeable (permeability is equal to water, cannot exert an osmotic effect against water)
= impermeable (permeability far less than water, will exert an impassible osmotic effect)
Urea across red blood cells is highly permeable (0). In this case, pi is 0, meaning no osmotic pressure.
Water movement
Hydrostatic pressure is the pressure against osmotic pressure due to weight of the water.
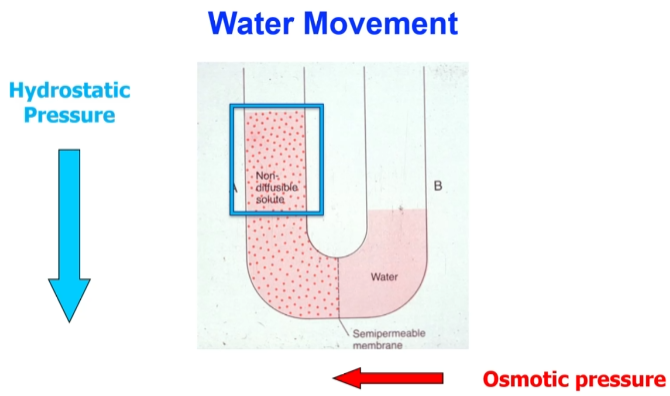
Increased osmotic pressure leads to higher flow and increased volume of the compartment. Putting pressure in the opposite end will force solute back into solution. This is called reverse osmosis.
Hypotonic: Low relative solution concentration as water moves in, cell will lyse (burst).
Hypertonic: High relative solution concentration as water moves out, cell will shrink.
Osmotic composition
Osmoles = # of active particles
If you were to take the Na/Cl/HCO/other concentration of plasma, you would get around 300 milliosmoles (mosm) per kg of water. There is no significant amount of protein in interstitial fluid, but the other groups will make up the difference. Same case for intracellular fluid.
See concentration breakdown for each compartment here: Body Fluid Compartments > Fluid volume
EXAMPLE
Why do red blood cells lyse when placed in water?SOLUTION
mmHg = osmolarity (300) x RT (19.3) = 5,790 mmHg
Volume of the compartment is determined by number of osmoles
As NaCl (solute) is withdrawn from a compartment, the compartment shrinks, causing water to rush in. This restores osmotic equilibrium.
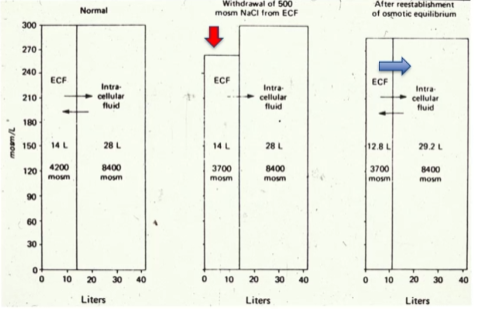
Loss of osmoles reduced the size of the ECF.
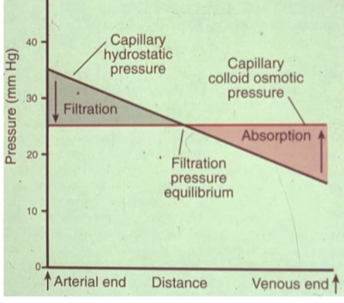
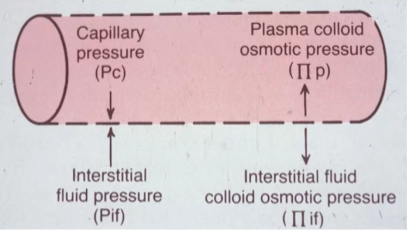
Pressures across a capillary
- Capillary pressure (Pc): Force pointing out. Comes from pumping of the heart.
- Interstitial fluid pressure (Pif): Force pointing in. Some pressure in interstitial fluid, although minor.
- Plasma colloid osmotic pressure (p): Force pointing in. Proteins (solute) pull water in.
- Interstitial fluid colloid osmotic pressure (if): Force pointing out. There are still some proteins (solute) in the interstitial fluid, although very minor.
Fluid net filtration pressure (Jv) = Kf x [(Pc - Pif) - (p - if)]
Kf is a constant
In the arterial end, capillary hydrostatic is high and fluid leaves the capillary (filtration). Nearer to the venous end, capillary hydrostatic pressure is lower than osmotic pressure and fluid enters the capillary (absorption).