Carbohydrates And Sugar Code Primer
Carbohydrates (sugars) follow the formula .
Sugar Nomenclature
Monosaccharides are named by number of carbons. Triose: 3C, tetrose: 4C, etc.
When ending in -ranose, it is a ring named similarly. Furanose: 5C ring, pyranose: 6C ring, etc.
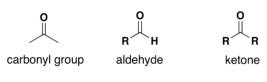
-aldehyde: C=O group at the end
-ketose: C=O group plus an extra item on the same carbon
Chirality
Most monosaccharides have at least 1 chiral carbon, creating 2 stereoisomers (D and L). The number of stereoisomers = 2^chiral centers . Epimers are stereoisomers which differ at 1 chiral carbon. Most natural sugars are D.
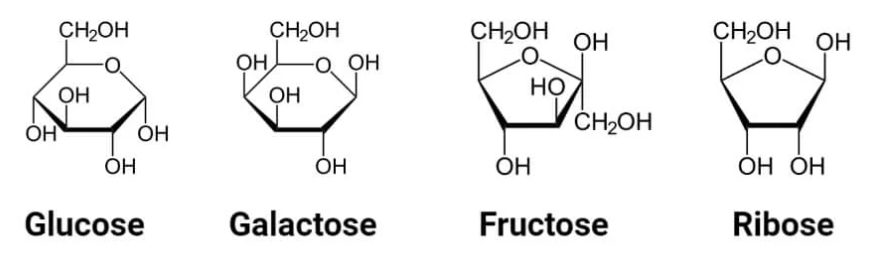
Anomeric carbon: Bound to two oxygens. Alpha has OH group on the bottom, beta on the top.
Mutarotation: Flipping between alpha and beta configurations until equilibrium is reached.
When sugars are in open form (the anomeric carbon is available to donate electrons), it can act as a reducing agent. Benedicts test will show if there are reducing sugars in urine. Blue = negative (no reducing sugars), red = positive (yes reducing sugars).
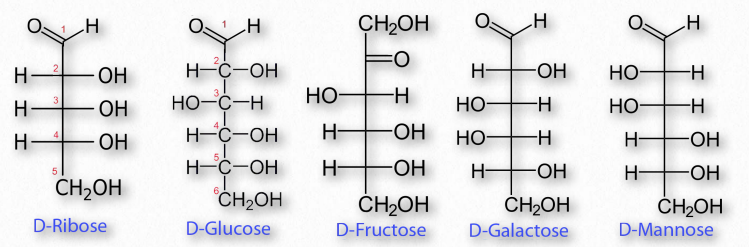
Important Monosaccharides
There are five simple sugar structures worth knowing: Ribose, Glucose, Fructose, Galactose, and Mannose.
Sugar Modifications
No modifications
Amino sugar
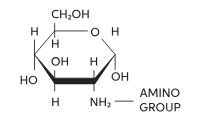
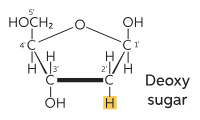
Deoxy sugars (deoxygenation)
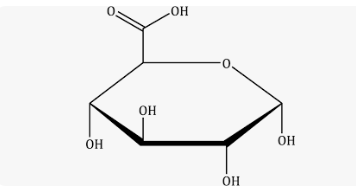
Carboxy sugars (carboxylation)
Other modifications
Aldonic acid: Oxidating aldose carbonyl
Uronic acid: Oxidation at opposite end
Lactone: Has an ester portion
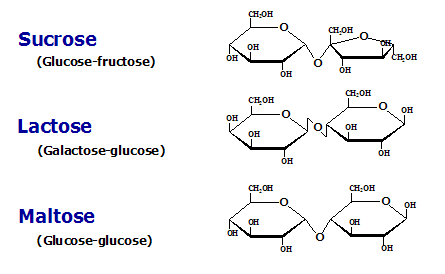
Disaccharides
2 monosaccharides linked via O-glycosidic bond, which is formed when hydroxyl of one sugar reacts with anomeric carbon of another. The non-reducing end donates its anomeric carbon while the reducing end retains its anomeric carbon.
Exception: Sucrose has both of its anomeric carbons react with each other. Thus, sucrose is non-reducing.
There are three digestible disaccharides: Sucrose, Lactose, and Maltose.
Polysaccharides
Functions
- Fuel storage (e.g. glycogen and starch)
- Structure (e.g. cellulose, chitin, glycosaminoglycans)
- Information transfer (e.g. sugar code)
Fuel storage
Glycogen has a chain of glucoses linked via alpha 1-4 bonds. It branches via alpha 1-6 bonds every 9-12 sugars on the chain. Glycogen only has one reducing end and several non-reducing ends. Glucose-1-P (G1P) can be released very quickly from the numerous non-reducing ends, making glycogen good for energy.
However, because glycogen is hydrophilic (will pull water into the cell), it can only be used for short term storage. Depletion of glycogen will occur within 12 hours of fasting.
Glycosaminoglycans
Makes up the EXM/ground substance of the extracellular matrix, which is a gel-like material between cells. Is has repeating saccharide pairs, an amino sugar, an acidic sugar (carboxyl), and is sometimes sulfated. It will covalently bond to proteoglycans. All of the negative charges make it play nice with water.
Sugar Code
Molecular messaging via the complexity of carbohydrates. Cells are recognized by surface glycans.
Selectins/lectins
Proteins which use the sugar code to recognize carb structures.
Selectins
Mediate inflammatory response in arthritis, asthma, psoriasis, multiple sclerosis, and transplanted organ rejection.
Influenza virus
- Hemagglutinin (lectin) [H]: Antigen on influenza virus recognizes a glycan on cell surface which is essential for infection
- Neuraminidase [N]: An enzyme which cleaves the glycan on the influenza virus surface to release the virus
The different H and N antigens year to year are which flu shots must be updated. Tamiflu and Relenza are sugar analogs which inhibit N
ABO blood groups
H/O antigen: Core of the glycan, Type O blood only has the H antigen (no A/B)
Type A: Enzyme adds N-acetyl galactosamine
Type B: Enzyme adds galactose