Ion Channels And Action Potentials
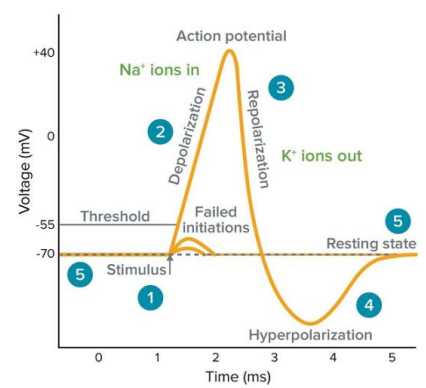
Anatomy of an action potential
I. Rest. Potassium leak channels maintain voltage
II. Stimulus → Opens voltage-gated sodium channels
III. Depolarization to -55 mV → Rapidly opens the rest of the sodium channels
IV. Peak → Voltage-gated sodium channels close, delayed rectifier potassium channels open
V. Delayed rectifier potassium channels still open → Hyperpolarization
VI. Delayed rectifier potassium channels close → Return to -70 mV
I. Rest. Potassium leak channels maintain voltage
Resting potential (5)
See here for details: Resting Potential > Resting membrane potential
At resting potential, there will be more potassium current than sodium because of leak channels. This sits around potassium's equilibrium potential (-70 mV). Sodium channels will be closed at this stage.
Stimulus (1)
With an outside stimulus, sodium influx begins and its inward current grows larger than potassium's outward current. At the threshold voltage (-55 mV), all sodium channels open and depolarization begins.
Depolarization (2)
With voltage-gated Na channels open the membrane rapidly becomes positive. As it crosses 0, this is referred to overshooting.
At the peak, voltage gated sodium channels will close and delayed rectifier potassium channels open.
Repolarization (3)
Potassium leaves the cell from delayed rectifier potassium channels and the membrane potential becomes more negative.
Hyperpolarization (4)
Rectifier potassium channels have a long lasting activation and will result in an undershoot of the target membrane potential.
Measurement clamps
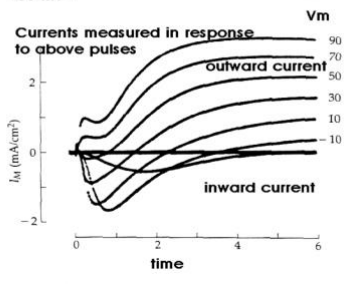
Voltage clamp
Holds the membrane at a fixed voltage which allows the current to be measured in the cell.
When reading a clamp diagram, negative current (down slope) represents positive ions entering the cell, whereas positive current (up slope) represent positive ions leaving the cell.
Current clamp
Keeps current constant so as to measure voltage.
Patch clamp
Newer variant of the voltage clamp which can measure the current in a single channel.
Channel blockers
Tetrodotoxin (TTX): Blocks voltage-gated sodium channels. Found in puffer fish.
Saxitoxin (STX): Block voltage-gated sodium channels. Found in "red tide" algae
Tetraethylammonium (TEA): Blocks voltage-gated potassium channels
Use of TEA with a voltage clamp allows for measurement of inward movement of Na during depolarization.
Comparing Na/K channels
| Voltage-gated Na Channels | (Voltage Gated) Delayed Rectifier K Channels | |
|---|---|---|
| Opening/Closing speed | Rapid | Slow, delayed |
| Inactivation period? | Yes | No |
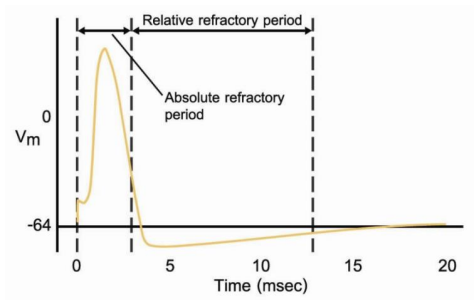
| Refractory type | Absolute | Relative |
| Action potential stage | Depolarization | Repolarization |
| Direction | In | Out |
Absolute refractory period: No action potential able to be generated
Relative refractory period: Yes action potential can be generated (but requires a stronger stimulus because of hyperpolarization)
Remember, voltage-gated Na channels are passive and open with the stimulus. They are not Na/K pumps.
Clinical pearls: Sodium channels
Hyperkalemic periodic paralysis: Sodium channels experience a point mutation in the transmembrane domain 4 (voltage sensor)
Anesthetics: Sodium channels are a target for lidocaine and cocaine
Local circuits
When depolarization occurs, the channels can open in either direction. Therefore, the refractory period of the sodium gates prevents the action potential from being able to propagate in the wrong direction.
The local potential (polarization) flowing through the neuron is dependent on the signal strength.
Calcium regulation
Increased extracellular Ca2+ will result in sodium channels being less sensitive to voltage changes and require a larger depolarization to fire. Decreased Ca2+ will allow sodium channels to open more readily and require less depolarization to fire. Low calcium could result in paralysis or death.
The process of increasing calcium in the cytoplasm to facilitate neurotransmitter release is referred to as excitation-secretion coupling. It helps vesicles dock at the presynaptic membrane for exocytosis.
Nodes of Ranvier
Unmyelinated sections of axon which contain voltage-gated sodium and potassium channels. In general, sodium channels are more dense in these nodes, which allows the action potential to jump over the myelin gaps, speeding up transmission. This is referred to as saltatory conduction.
Potassium channels will be more concentrated in the perinodal axolemma.
Source of myelin
Central Nervous System (CNS): Oligodendrocytes
Peripheral Nervous System (PNS): Schwann cells
Loss of myelin will result in multiple sclerosis (MS) and Guillain-Barre syndrome (PNS)
Conduction velocity
Measures the speed of the action potential down the axon. Large space constant and small time constant maximizes this.
Myelin conductive mechanism
- Increases resistance
- Decreases axonal diameter
- Decreases current loss to environment (increasing capacitance)
Space constant =
Rm = membrane resistance
Ri = internal resistance (inverse of axon diameter)
Increasing membrane resistance (via properties of phospholipids, leak channel concentration, myelination, etc..) or the axon diameter will increase the space constant.
Time constant =
Rm = membrane resistance
C = membrane capacitance
Capacitance is the key factor here. Decreasing capacitance reduces the stored charge, reducing the time constant, and increasing conduction velocity.