Signal Transduction Primer
Signal classification
Autocrine: Signaling the same cell
Paracrine: Neighboring cell
Endocrine: Distant cell (via circulation)
Not all ligands bring about signal transduction. Those which do not transduce a signal are antagonists.
Binding
Dissociation constant
Receptor (R) + Ligand (L) → RL
Velocity of formation = k1 [R][L]
Velocity of dissolution = 1/k1 [RL]
At eq., the velocity of formation and dissolution is the same
Lower dissociation constant (Kd) = the tighter binding
Signal specificity
Receptor distribution
Hepatocytes express glucagon receptor at high levels. Therefore they respond to glucagon. By contrast, myocytes do not express glucagon, so they do not respond to glucagon. Myocytes instead express b-adrenergic receptor so they respond to epinephrine.
Downstream effects
The same receptor can have different downstream effects. acetylcholine (g-protein coupled) promotes skeletal muscle contraction, but inhibits smooth muscle contraction.
Second messengers
A primary messenger will bind to a membrane ligand but cannot enter the cell. This will promote release of the second messenger to do work within the cell.
Calcium signaling
Ca2+ is much lower in the extracellular conc. because of pumps. Protein kinase C (PKC) is a common target.
Calmodulin also binds to Ca2+ to mediate downstream effects. When calmodulin binds calcium, the whole molecule wraps around target proteins. These can go activate calcium-calmodulin-dependent protein kinases.
IP3 and DAG
Phosphatidylinositol 4,5 bisphosphate (PIP2) cleavage yields IP3 and DAG. This is activated by Phospholipase-C.
Note the cleavage site:
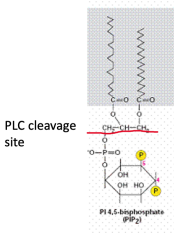
DAG will stay in the membrane where is binds. IP3 is soluble and can activate other downstream targets (e.g. gated calcium channels).
Cyclic nucleotides
AMP (cAMP) is generated by adenylate cyclase. AMP binds PKA.
Cyclic GMP (cGMP) is generated by guanylyl cyclase. cGMP binds PKG.
They may also bind to and activate other proteins.
Guanine binding proteins
Heterotrimeric binding proteins
Trimeric (3) G-binding proteins have alpha, beta, and gamma subunits.
Activation
- alpha is initially bound to GDP and associated with the beta/gamma subunits
- GDP is released and GTP is bound (GTP/GDP exchange)
- alpha is released to initiate downstream events
Inactivation
5. GTPase turns off the signal and rebinds alpha to beta/gamma
Classes of G-proteins
- Gs alpha: Stimulates adenylate cyclase (s for stimulate)
- Gi alpha: Inhibits adenylate cyclase (i for inhibit)
- Gq alpha: Act through phospholipase C (yields IP3 and DAG). Seen in histamine receptors (q for quirky)
Adenylate cyclase increases cAMP which activates PKA.
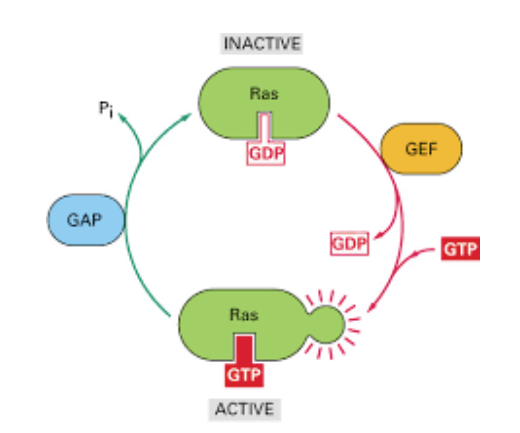
Small GTP binding proteins
Single subunit proteins. Active when bound to GTP. Ras has inherent GTPase activity and will cleave GTP to GDP, deactivating the protein.
Activation
- Inactive when bound to GDP
- A guanine exchange protein (GEF) will cause Ras to release GDP
- GTP binds to activates it.
Inactivation
- GTP is then cleaved back to GDP via GAP
Kinase cascades
Kinases phosphorylate via ATP. This occurs on either tyrosine serine/threonine residues. Most are specific for Y or S/T. Kinases often have the effect of amplifying the signal as it can phosphorylate multiple targets.
Receptor types
G-Protein coupled receptors (or seven transmembrane receptors)
Most common target for pharmaceuticals
- Can release IP3, DAG and Ca2+ via phospholipase C (PLC).
- IP3 will increase Ca2+ via the ER
- DAG will activate PKC
- Can also trigger the Mitogen Activated Protein Kinase (MAPK) cascade. This will trigger cellular proliferation. Vocab: A mitogen is a substance that triggers cell proliferation
Receptor tyrosine kinases
Ligand binding domain in the streets (extracellular), tyrosine kinase domain in the sheets (intracellular). Types include insulin receptor and growth hormone receptors.
- Hormone binds receptor on cell surface
- Receptor dimerizes and activates via autophosphorylation on Y residue
- Active receptor has down stream effects via kinase cascade
That single signal can have multiple interval responses. RTKs are often linked to the activation of small GTP binding proteins like ras.
Guanylyl cyclase
Mostly for fluid regulation (e.g. atrial natriuretic peptide (ANP) receptor). The guanylin receptor of the intestine, is the target of bacterial endotoxin.
- Ligand binding leads to dimerization
- Guanylyl cyclase is activated
The NO-receptor form can pass right through the cell membrane and does not have to be on the cell surface.
Adhesion receptor: Integrins
Alpha and beta subunits will undergo conformational change to high affinity binding site upon agonism. Can also respond to the extracellular matrix (ECM) of other cells.
Integrin signaling is bidirectional. Outside-in signaling alerts the cell an ECM was encountered. and promoted contact inhibition Inside-out signaling will cause cell adhesion via the talin protein.
Conformations:
- Low affinity: No signaling
- Intermediate affinity: One type of signaling present
- High affinity: Both types of signaling present
Platelets make use of the high affinity conformation to form clots.
Notch receptor
Important in embryonic development and inhibition leads to impairment for Alzheimer's patients.
- Notch receptor binds notch ligand on surface
- Notch receptor undergoes intramembrane cleavage
- Intracellular notch activates genes in nucleus
Nuclear Receptors: Androgen receptor
A target of fat soluble hormones, travels straight through membrane. These bind hydrophobic hormones which diffuse through the cell membrane.
Type I: Bind in cytoplasm and move into nucleus
Type II: Always reside in nucleus and becomes active upon binding of hormone
Signal integration
Transduction and amplification
When a signal is linear, one messenger elicits on response. However, when a signal is amplified a given molecule of A might go on to activate more than one of B which could go on to activate more than one of C, multiplying the effect.
Example: Muscle contraction
When muscle cells gets the signal to contract, there is a spike in Ca2+ in the cell. Binding of acetylcholine depolarizes the membrane and releases Ca2+ from the sarcoplasmic reticulum. Calcium changes the configuration of the troponin which exposes binding sites of actin. At the same time, it stimulates phosphorylase kinase which increases glu-1-p from glycogen and ATP production.
This is an example in which two pathways sharing a receptor/effector converge on the same node. This is known as node convergence.
PROBLEM
The cholera toxin enters enterocytes in the GI tract and modifies the Gs alpha subunit, preventing it from hydrolyzing GTP. What is the result of this?SOLUTION
Gs alpha activates adenylate cyclase
- Less adenylate cyclase would increase cAMP
- More cAMP increases CFTR ion channel activity
- More CFTR ion channel activity increases sodium and chloride efflux
- Water then flows into GI lumen, creating watery diarrhea and dehydration