Fertilization And Implantation
Menstrual Cycle
1. Early follicular phase (mensus phase in uterus)
- Pituitary releases follicle stimulating hormone (FSH), recruiting 10-20 primary follicles in the ovary. One follicle is selected.
- The selected follicle secretes estrogen, which inhibits FSH through negative feedback and causes endometrial proliferation
2. Mid follicular phase (proliferative phase in uterus)
- The follicle continues to grow and secrete more estrogen. Once it reaches an estrogen level of 150 pg/mL for 36 hours, the negative feedback becomes positive and luteinizing hormone (LH) surges.
- When LH surges, it induces the follicle to secrete progesterone and induces ovulation
Oocytes pre-ovulation cannot be fertilized. The egg post-ovulation can be fertilized. The egg is paused in meiotic metaphase II
3. Secretory phase (luteal phase in ovarian cycle)
- Remnants of the ovulated follicle (corpus luteum), secretes estrogen and progesterone
- Lots of progesterone inhibit FSH
- FERTILIZATION DOES OCCUR: Corpus luteum remains intact and continues to secrete progesterone. Human chorionic gonadotrophin (hGC) from syncytiotrophoblasts maintains corpus luteum
- FERTILIZATION DOES NOT OCCUR: No hCG means corpus luteum degenerates, decreasing progesterone/estrogen, increasing FSH and mensus
How to prevent pregnancy (very important)
Pre-ovulation
Levonorgestrel (Plan B) is synthetic progestin. Progestin upregulates progesterone, which downregulates FSH, resulting in no follicular development (no recruitment). No follicular development which means no LH surge/no ovulation
Post-ovulation
Ru486 (mifepristone) is a competitive antagonist at progesterone receptors. The uterus, therefore, thinks there's no progesterone present and FSH is not inhibited
Fertilization
- Sperm lose their glycoprotein coat, allowing for active forward motion. In this case, the sperm head also undergoes molecular changes allowing it to bind to the egg
- Sperm finds the egg in the oviduct, usually within the ampulla
- Sperm penetrates the corona radiata (made of granulosa cells), signaling the acrosome reaction to occur
Acrosome reaction
- Acrosomal membrane of the sperm breaks down and releases hyaluronidase, which eats away at the hyaluronic acid holding together the corona radiata.
- Sperm can then bind to the zona pellucida (egg shell), which is composed of 3 glycoproteins (ZP1, ZP2, and ZP3)
- Acrosin released during the acrosome reaction eats the glycoproteins
- The egg/sperm fuse when the sperm membrane protein fertilin binds to integrin on the egg plasma membrane. But we're not sure if this is the case.
cont:
- Sperm fusion initiates waves of Ca2+ out from the entry point and then oscillations of intercellular concentration. This causes the cortical granules (vesicles underneath egg plasma membrane) to exocytosis their contents (cortical reaction).
Cortical reaction
Exocytosis of granule content causes the zona pellucida to harden and blocks polyspermy. This achieved via three things:
- The ZP3 glycoprotein is inactivated and will no longer bind to sperm
- The ZP2 glycoprotein is cleaved, hardening the zona pellucida
- The Ca2+ wave activates protein kinase C (PKC) and Calcium-calmodulin-dependent protein kinase II (CaMKII), which causes the egg to resume meiosis
cont:
- After resuming meiosis, the male and female pronuclei (23 chromosomes each - haploid), replicate their DNA in the S phase
- The pronuclei membranes dissolve and the chromosomes pull apart in metaphase to create a diploid zygote with 46 chromosomes. Abnormal separation of chromosomes is the most common cause of spontaneous abortions. This is also the cause of trisomy 21 (down syndrome).
Blastocyst formation
Totipotent cells: Can give rise to any cell, including the placenta
Pluripotent cells: Can give rise to any cell, except the placenta
Blastomeres: Round cells within embryo which divide by mitosis
For more on stem cells, see here: Stem Cells
1. Induction
With our first diploid cell, we now have the zygotic genome. The cells split in two, embryonic genes turn on, and maternal genes turn off
2. Morula (3 - 4 days)
At the 12-cell stage, blastomeres express adhesion junctions (adherens, desmosomes, tight junctions) which allows them to flatten on top of each other
There are 11 outside cells and 1 inside cell. The outside cell sees the inside and outside while the inside cell sees only sees the inside environment. This allows the cells to differentiate.
The outer cells (trophectoderm) express Na+/K+ ATPases. As bananas are pumped into the interstitial fluid of the embryo, water follows and forms a fluid filled cavity (blastocele)
3. Blastocyst (4.5 - 5 days)
The blastocele is formed and pushes the inner cell mass to one side. At this point the inner cell mass is not differentiated, but the trophectoderm is.
4. Blastocyst hatches (5.5 - 6 days)
Implantation
1. Adhering to uterine wall
At this point, the blastocyst is hanging out in the uterus. The trophectoderm attaches to the endometrial epithelium (one-cell thick) at the embryonic pole. The trophoblast cells first express selectins which loosely attach, than express integrins which tightly attach.
The blastocyst then secretes metalloproteases (MMPs) that eat the uterine epithelium. The cell then moves towards the decidua.
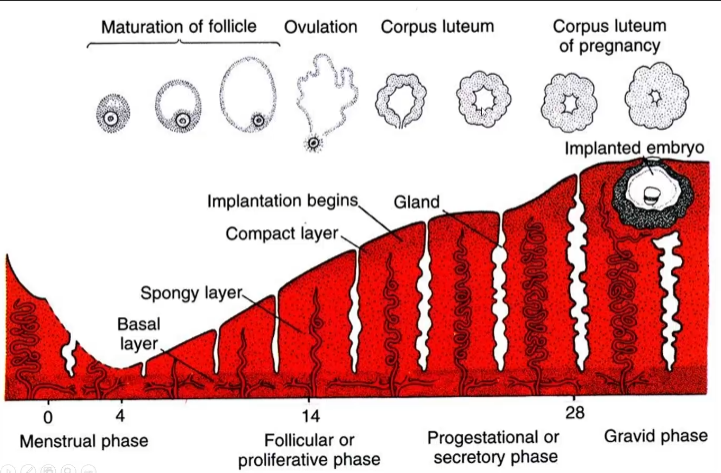
Three layers of endometrium:
- Compact layer: Most superficial, sloughed off
- Spongy layer: Middle layer, sloughed off
- Basal layer: Deepest layer (has its own blood supply), remains
2. Reaching decidua (5 - 9 days)
Upon reaching the decidua, the trophectoderm differentiate into into syncytiotrophoblasts (STB) and cytotrophoblasts (CTB). The trophoblast cell membranes fall apart and form a syncytium which becomes STB.
RECALL: STB is secreting hCG to maintain the corpus luteum which will secrete progesterone to keep the endometrium thick and full of blood vessels.
The inner cell mass differentiates into epiblasts (primitive ectoderm) and hypoblasts (primitive endoderm). The epiblasts migrate along the CTB to form the amniotic cavity. The hypoblasts grow along the CTB which forms Hauser's membrane. The STB surrounds the embryo.
3. Formation of extraembryonic mesoderm (8 - 12 days)
Hauser's membrane secretes extracellular matrix (ECM) materials (fibronectin, laminin, etc..). This forms an extraembryonic reticulum between Hauser's membrane and the CTB.
The STB produces digestive enzymes which eat away at capillaries. Blood pools as a result and form lacunae, the start of embryonic blood flow providing the embryo with nutrients.
The epiblast divides and migrates between Heuser's membrane and the CTB, forming the extraembryonic mesoderm. As this mesoderm surrounds Heuser's membrane, it can no longer secrete ECM materials, breaking the extraembryonic reticulum, and forming a fluid filled cavity called the chorionic cavity.
4. Yolk sacs (12 - 13 days)
The extraembryonic mesoderm differentiates further into the primary yolk sacs. The secondary yolk sac then forms and the primary yolk sac pinches off and disintegrates.
Blood vessels form first in secondary yolk sac and then, after a day, in the extraembryonic mesoderm.
5. Bilaminar disc formed within chorionic cavity
Clinical peals
Ectopic pregnancies
When implantation occurs outside the uterus. The most common site is the ampullary. Characterized by rupture of blood vessels, vaginal bleeding, abdominal pain. May require removal of embryo.
Pre-eclampsia
Occurs in 5% of all pregnancies and accounts for 20% of serious birth complications. Cause not well understood, but is genetic.
Pathophysiology:
- ↑ sFLT-1
- VEGF receptor (vascular endothelial growth factor) on epithelial cells. Responsible for blood vessel growth. Will circulate in blood as sFLT-1 normally.
- ↓ PLGF
- Placental growth factor. Produced in placenta and binds to FLT-1 to enhance blood vessel growth (esp. 3rd trimester)
Too much sFLT-1 will soak up available PLGF (and VEGF) so it wont be able to bind to FLT-1. If FLT-1 doesn't bind it causes blood vessels will deteriorate and inhibits growth of new blood vessels in placenta and other organs.
Treatment: Flood mother with PLGF to bind to FLT-1.
Symptoms:
- Hypertension
- Proteinuria
- Liver inflammation
- Edema
- Platelet depletion
- Premature birth
- Severe cases: eclampsia (swelling in brain/convulsions, coma, death)