T Cell Mediated Immunity
Secondary Lymph Organs
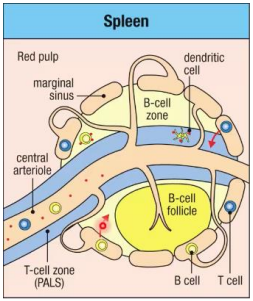
Spleen
Lymphocytes enter from the blood via the marginal sinus. T cells move to the T cell zone while B cells move to the B cell follicle.
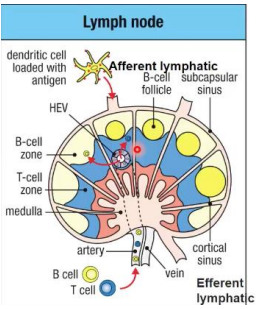
Lymph nodes
Naive T cells arrive at lymph nodes through the blood and enter via the high endothelial venule (HEV). They then move into the paracortex (T cell zone).
Activated dendritic cells enter via the afferent lymphatic. The move to the T cell zone
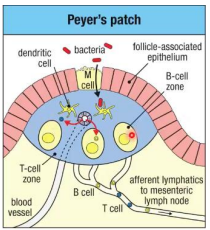
Peyer's Patches
Naive T cells arrive at Peyer's Patches through the blood and enter via the high endothelial venule (HEV). Activation does not occur in Peyer's Patches and will move to the lymph node for activation.
Dendritic cells arrive via the lumen.
Entry into Tissues
1. Rolling
Naive T cells in HEV express lectins which interact with L-selectin, allowing T cells to roll on the surface.
2. Activation
Chemokine receptors (CCR7) on T cells recognize CCL21 on HEV, changing conformation to bind integrins on the T cells. The T cells can then enter the lymph node paracortex, following the cytokine gradient.
Survival Signals
T cells which do not encounter an antigen in the lymph node paracortex receive a survival signal, or a signal from the host peptide which keeps the T cell alive in the lymph node indefinitely.
When there is a TCR/dendritic cell antigen match in lymph nodes, MHC triggers a confirmational change in LFA1, leading to a stronger interaction. This leads to clonal expansion over 2-3 days.
Exit from Tissues
Controlled by sphingosine 1-phosphate (S1P) which is in high concentration in lymph and blood. Lymph nodes keeps the concentration low internally using S1P lyase.
Without an antigen encounter, S1PR1 (a GPCR for S1P) is upregulated and T cells will follow the S1P gradient to the plasma.
If an antigen is encountered, CD69 is upregulated which results in proliferation of effector (activated) T cells. Eventually CD69 is downregulated and S1PR1 is upregulated, which suggests to the T cell it is time to leave.
Clinical pearl: FTY20
FTY20 inhibits immune response by trapping lymphocytes in the lymph node. This is a useful therapy for multiple sclerosis, chronic infections (e.g., HIV/SIV), and cancer.
T Cell Activation
T cells receive three types of signals, all three of which activate the T cell.
- Specificity for TCR (ability to recognize MHC antigen)
- Co-stimulatory signal (amplifies signal 1)
- Cytokine signaling
Upon activation, T cells switch from oxidative phosphorylation to aerobic glycolysis.
Signaling Cascade
After activation, resulting in clonal expansion. After the threat is eliminated, effector (activated) T cells are removed (clonal contraction) and those remaining become memory cells.
Akt: Switches T cell to anerobic glycolysis
Il-2: Growth factor sustaining clonal expansion
Il-2R alpha: Allows T-cells to respond to low IL-2
CD69: Receptor for lymph node retention.
IL-2
A growth factor for sustaining clonal expansion. Its receptor (IL-2R) responds to low concentrations of IL-2. This is important for T regulatory cell survival.
Control of Clonal Expansion
Inhibitory receptors (checkpoint receptors). Are sometimes used for cancer therapy.
CTLA4: Restricts activation of T cells through higher affinity for B7 than CD28.
PD1: Binds Programmed Death Protein Ligand 1 and 2 (PDL1 and 2).
T Cell Differentiation
CD8 Cytotoxic (Killer)
Defends against intracellular pathogens (e.g. viruses). Requires co-stimulatory activity.
CD4 cells, activated by dendritic cells, help CD8 expansion through IL-2 secretion.
CD4 Helper
Cytokines induce the expression of transcription factors for T cell subtype differentiation via the JAK-STAT pathway, upregulating master regulator genes which control what the T cell secretes.
Master Regulator Genes
TH1: STAT1 (IFN-gamma), STAT4 (IL-12) → T-BET
TH2: STAT6 (IL-4) → GATA3
TH17: STAT3 (TGF-beta, IL-6, IL-23) → BCL6
TFH: STAT5 (IL-2) → FOXP3
Secretions
TH1: Produces IFN-gamma (Macrophage mediated destruction of pathogens) and IL-2 (T-Cell Growth Factor)
TH2: Produces IgE (parasites), as well as IL-4, IL-5, and IL-13
TH17: Produces IL-17 (neutrophil recruitment) and IL-22 (stimulates antimicrobial peptide release)
TFH: Produces IL-21 (isotype switching)
T-reg: Produces IL-10 and TGF-beta (mucosal homeostasis and T cell response regulation)
Dendritic Cells (DCs)
The main goal of these is to activate naive T cells. Upregulation of CCR7 receptors on DCs with antigen presentation cause their migration into the lymph node.
Conventional DCs
Detects a variety of pathogens.
cDC1 is specialized in activation in CD8 T cells
cDC2 is specialized in activation of CD4 T cells
Plasmacytoid DCs
Detects specifically viral infections.
They express TLR-7, TLR-9, and IFN-alpha which help produce type I interferons (anti-viral cytokines).
Antigen processing
Receptor-mediated phagocytosis
Pathogen engulfed after MHC II presentation. Activates CD4 T cells.
Viral infection
Degraded by cytoplasmic proteosomes. Peptide loaded onto MHC I. Activates CD8 T cells.
Cross-presentation
When the virus has not directly infected the cell, but still contacted. Peptide loaded onto MHC I. Activates CD8 T cells.
Transfer between DCs
Pathogen is exchanged between DCs. This is used for self-antigens and will be presented on both MHC I and MHC II. Activates both CD8 and CD4 T cells.
Autophagy
When naturally recycling organelles, internally created peptides are presented on MHC II. This maintains tolerance to self-antigens.