Muscles
Skeletal Muscle
Structure
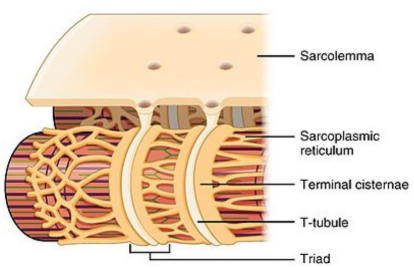
Fibers are composed of myofibrils and covered by a sarcolemma. The sarcolemma invaginates to create structures called transverse tubules (t-tubules), which are grooves in the fibers that action potentials can use to innervate deeper tissue.
Terminal cisternae border t-tubules and contact the sarcoplasmic reticulum (SR). The SR, analogous to the endoplasmic reticulum (ER), will store calcium using calsequestrin.
Triads are a group of two terminal cisternae with a bisecting t-tubule. They are important for excitation-contraction coupling. Depolarization will move from t-tubule → terminal cisternae → SR.
Action potential mechanism
The end plate will trigger an action potential which spreads down the muscle fiber. This will proceed normally, with the one caveat that potassium cannot fully clear from t-tubules because of lack of space. With high potassium, repolarization is temporarily inhibited. The gradient is reestablished using chlorine channels which are always active.
Clinical pearl: Muscle fatigue
Accumulation of extracellular potassium may be the cause of muscle fatigue. Preventing fatigue can be accomplished by boosting the chlorine leak channel, which can be achieved with lactic acid. Myotonia is a genetic disorder targeting this leak channel (downregulating them).
Mechanical contraction
With depolarization, L-type calcium channels undergo a conformational change. L-type channels will activate ryanodine receptor channels, which allow calcium to be released from the SR into the sarcoplasm. Calcium activates troponin C, which leads to contraction.
Depolarization → L-type calcium channels → ryanodine receptor channels → troponin C → contraction
Sliding filament structure
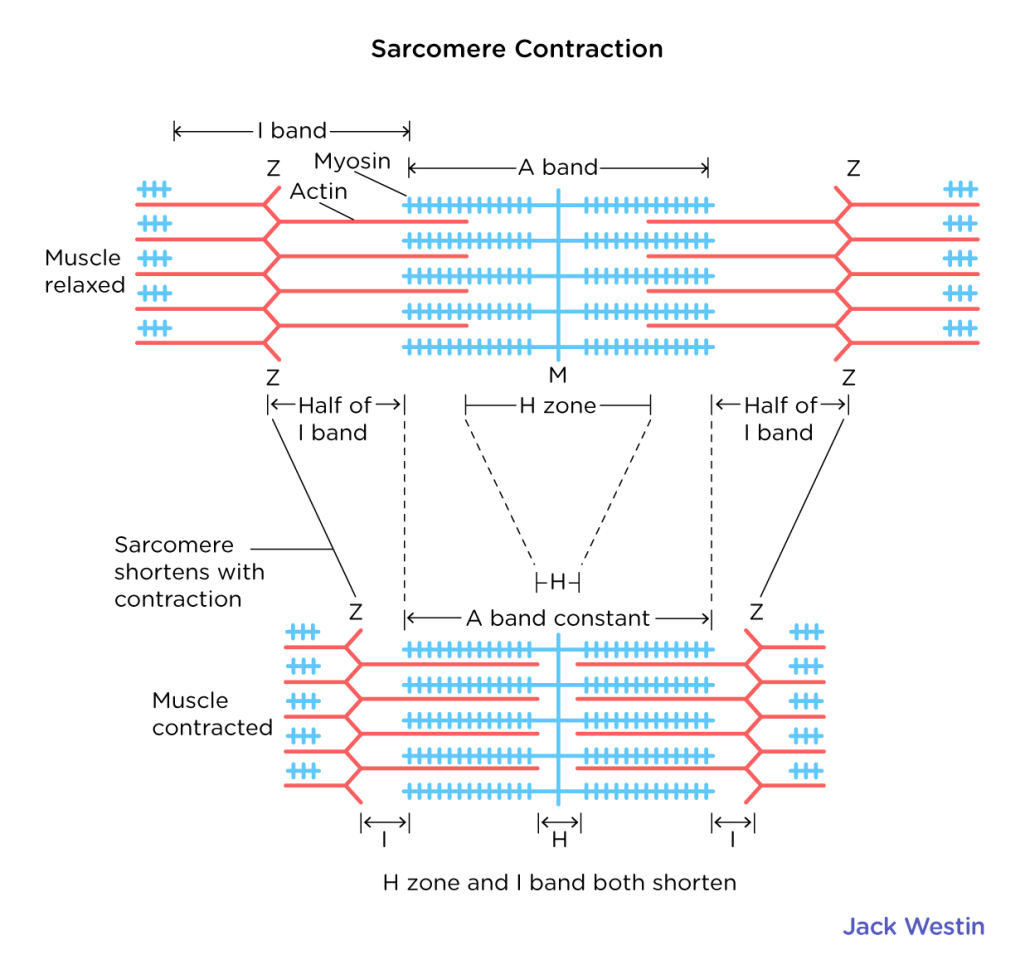
Myofibers contain sarcomeres, a single working muscle unit. These are composed of actin (thin filament) and myosin (thick filament). When the muscle contracts, actin uses myosin as the static anchor to move.
A band: The myosin (thick). Stays the same length when contracting
I band: The actin (thin). Will shrink with contraction
H band: The span of myosin between the I bands. Will shrink with contraction
A band: The myosin filament (thick). Stays the same length when contracting
I band: The actin filament (thin). Will shrink with contraction
H band: The span where there is only myosin and no actin between the I bands. Will shrink with contraction
Z line: Attachment point for actin filaments. The area between Z lines is one sarcomere.
M line. Attachment point for myosin. The midpoint of the sarcomere.
Sliding filament theory
G-actin monomers form an F-actin (functional) filament. Two F-actin filaments form a functional actin filament.
Myosin doubles up on everything
- Two heads: One binding ATP, one binding Actin
- Two chains of light meromyosin
- Two chains of heavy meromyosin
- Two hinged regions
When ATP binds, the hinged regions on myosin will bend 45-degrees, referred to as a power stroke.
Filament binding regulation
Tropomyosin is found along the length of an actin filament. It must be moved to bind myosin.
Troponin contains three subunits
- Troponin-T (T for aTTach): Attaches troponin to tropomyosin
- Troponin-I (I for inhibit): Locks tropomyosin in place and inhibits myosin
- Troponin-C (C for calcium): Binds calcium, which moves tropomyosin and allows actin to bind myosin
Cross-bridge cycle
- Resting State: Myosin head is loaded with ATP, and tropomyosin covers binding sites on actin. Troponin-T attaches the troponin complex to tropomyosin and Troponin-I holds tropomyosin in position.
- Calcium Release: Action potential triggers the release of calcium ions from the sarcoplasmic reticulum.
- Calcium Binding: Calcium ions bind to Troponin-C causing Troponin-I to release tropomyosin, exposing the actin binding sites.
- Cross-Bridge Formation: Myosin binds to actin.
- Power Stroke: ATP hydrolysis causes myosin head to pull the actin filament.
- New ATP Binding: Myosin releases ADP and phosphate and a new ATP molecule binds.
- Cross-Bridge Detachment: ATP binding causes the myosin head to detach from actin.
- ATP Hydrolysis: ATP is hydrolyzed, resetting the myosin head to its high-energy state, and the cycle can repeat.
The velocity of this cycle depends on the speed of ATPase, while the tension of the contraction depends on the number of cross-bridges formed and the concentration of ATP/calcium. Creatine will provide extra phosphate for ATP binding to improve this cycle efficiency.
Clinical pearl: Rigor mortis
When there is no remaining ATP (e.g., in a cadaver), the actin and myosin remain linked and the muscle remains contracted
Isometric and Isotonic Mechanisms
Isotonic contraction: Muscle tension remains constant, but velocity changes (lifting a weight)
- Concentric: Muscles shortening (curling)
- Eccentric: Muscle lengthening (reaching)
Isometric contraction: Muscle length remains constant, but tension changes (pushing against a static object)
The maximum velocity occurs when the muscle is under minimum load. The maximum tension occurs when the muscle is at its minimum length.
Active tension: Driven by the metabolic movement of muscle
Passive tension: Driven by the inherent flexibility of the muscle fiber. Highest when compressed
Getting swol
Hypertrophy: Increase in the number of sarcomeres and length of muscle fibers. The number of fibers does not change.
Hyperplasia: Increase in the number of fibers (this does not occur with training, e.g. pathological).
Regulation of muscle tension
A single action potential will create a twitch (contractile response). However, the series of elastic elements in muscle (tensons/tissue/fibers) will have some slack and prevent maximal contraction. Therefore, multiple action potentials will create multiple twitches, leading to a tetany or sustained, smooth contraction as all the slack is used up.
A motor unit consists of an alpha motor neuron attached to the muscle fibers it innervates. The innervation ratio is the muscle fiber / motor neuron ratio within the motor unit. A higher ratio is good for heavy tasks while a smaller ratio is good for precise tasks.
Amazingly, the brain will recruit the correct size motor unit for the task at hand.
Motor unit types
| Type 1 | Type 2 | |
|---|---|---|
| Color | Red | White |
| Fuel | Fat | Glucose (to lactic acid) |
| Aerobic? | Yes | No |
| Speed | Slow | Fast |
| Fatigue | Less | More |
| Function | Endurance | Power |
| NOTE: There is also Type 2a which has hybrid 1/2 properties |
Smooth Muscle
The difference between smooth muscle and skeletal muscle is three fold:
- Thick filaments are thinner
- Thin filaments are attached to structures called dense bodies
- No sarcomeres (no muscle striation)
Also, it is controlled by the autonomic nervous system whereas the skeletal muscle is controlled by the somatic nervous system.
Multiunit smooth muscle
Stimulation required from nerves for contraction (neurogenic). Used for fine motor controls, such as ciliary muscles and pili erector muscles for hair follicles. These cell membranes will be electrically isolated, with low density fibers.
Unitary (visceral) smooth muscle
Can contract without neural stimulation (myogenic). However, can still be innervated by nerve input. Is used for synchronous contraction and will often be connected by gap junctions to share electrical potential. A group of cells connected by gap junctions is called a syncytium.
Examples include the heart, GI, blood vessels, uterus, and bladder.
Action potential behavior
There are three types of responses to an action potential in smooth muscle
- Spike: Large depolarization. Can be from electrical stimulation, hormone, or muscle stretch.
- Plateau: Delayed repolarization allowing for smoother contraction. See bladder, esophagus.
- Slow waves: Highly extended repolarization allowing for rhythmic movements as a result of slow closing of voltage gated calcium channels. See stomach, intestines.
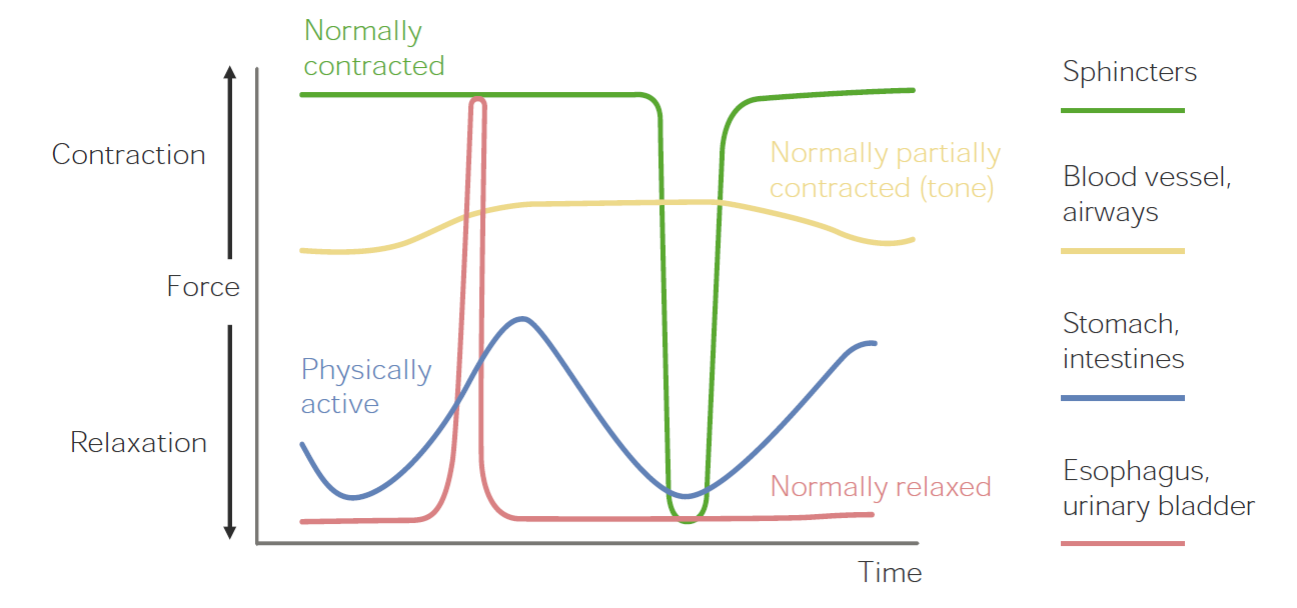
Note: Sphincter muscles exhibit the latch mode of contraction described below
Calcium transport
Voltage (L-type) gate: Located in caveoli and are triggered by an action potential
IP3 gate: Open in response to hormones or neurotransmitters
Calcium-induced calcium release channels: Calcium release from SR
Store-operated channels: Calcium influx from extracellular space
Contraction
Smooth muscle uses a calmodulin-binding mode similar to the cross-bridge system, without the troponin or tropomyosin regulation. The difference is that calmodulin is used to activate myosin via phosphorylation. Because calcium does not bind directly, smooth muscles are slower and less efficient.
- Ca2+ binds calmodulin
- Calmodulin binds myosin light chain kinase (MLCK)
- Myosin is phosphorylated by MLCK
- Cross-bridge formed with actin
- Myosin light chain phosphatase (MLCP) removes the phosphate, disconnecting the cross-bridge
Smooth muscle also has a latch mode of binding which allows it to maintain permanent contraction with minor energy expenditure. This is similar to the calmodulin mechanism, except after MLCK dephosphorylation of myosin a latch is formed with actin, locking it in place. You can see this in sphincter muscles.